Abstract
Introduction
The phase 3, randomized RAY study demonstrated significant improvement in progression-free survival (PFS) with ibrutinib versus temsirolimus in patients with relapsed or refractory (R/R) mantle cell lymphoma (MCL) (NCT01646021; Dreyling M, et al. Lancet. 2016;387:770-778). No head-to-head comparisons are available yet between ibrutinib and other widely used MCL treatments in clinical practice. The aim of this analysis was to investigate the relative treatment effect on PFS and overall survival (OS) of ibrutinib versus other treatments used in real-world (RW) clinical practice, using patient-level data (PLD) from RAY and the Lyon-Sud University Hospital database.
Methods
The regional, single-center Lyon-Sud University Hospital database in France holds electronic medical records for patients with MCL who were diagnosed between 1990 and 2016. Patients with R/R MCL were receiving second- (n = 106), third- (n = 57), or later-line (n = 88) treatment. PLD were cleaned and validated before analysis. PFS and OS were compared between ibrutinib and RW (physician's choice) treatment in R/R MCL using an adjusted comparison of PLD from the ibrutinib arm of RAY and Lyon-Sud (excluding ibrutinib RW patients). For the definition of PFS, missing data for progressive disease date for patients in Lyon-Sud who initiated subsequent therapy were replaced by the conservative proxy of initiation date for next treatment. Differences in patient baseline characteristics between the RAY trial population and the RW cohort (due to lack of randomization) were adjusted for by using a multivariate Cox proportional hazards model to estimate the hazard ratio (HR) for ibrutinib versus RW treatment. Age, sex, disease stage, and line of therapy were included as covariates. Curves predicting PFS and OS for the RW cohort, if they would have been exposed to ibrutinib, were derived from the multivariate model.
Results
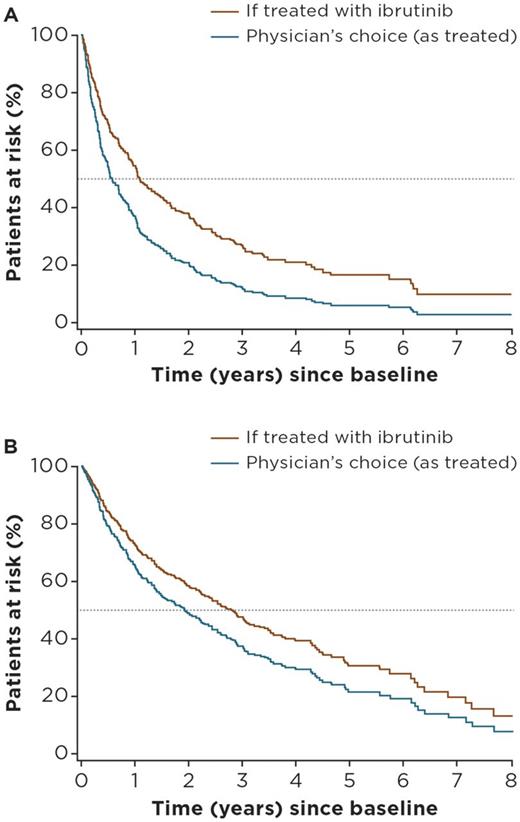
Median age at treatment initiation was 67 years, and the proportion of male patients was 75% in Lyon-Sud (n = 251), compared with 67 years and 72% in the ibrutinib arm of RAY (n = 139). Median follow-up was 61 months for Lyon-Sud versus 39 months for RAY. The most frequently used (> 75%) treatment regimens in the RW cohort were rituximab + chemotherapy (R-chemo; n = 67), chemotherapy (chemo, n = 54), cytosine arabinoside-based chemotherapy (AraC, n = 42), and targeted agents (n = 28). More information on these treatment groupings will be included in the final presentation. Advanced disease stage and later lines of therapy were independent risk factors for PFS and OS. Adjusted HRs for ibrutinib versus RW regimens (physician's choice) were 0.59 (95% confidence interval [CI]: 0.46-0.75) for PFS, and 0.74 (0.51-1.05) for OS. Adjusted HRs for ibrutinib versus R-chemo, the most commonly used regimen in the RW cohort, were 0.56 (0.40-0.79) and 0.83 (0.52-1.32) for PFS and OS, respectively. Adjusted HRs for ibrutinib versus AraC were 0.58 (0.40-0.84) and 0.63 (0.39-1.00) for PFS and OS, respectively. Predicted survival curves based on PLD from Lyon-Sud are presented in Figure 1A and B.
Conclusions
This adjusted comparison of outcomes from the RAY clinical trial of ibrutinib and physician's choice treatments in the RW setting augments growing evidence that ibrutinib improves PFS and OS versus commonly used regimens. The analysis suggests 1.7-fold improvement of PFS and 1.3-fold improvement of OS for ibrutinib compared with standard therapies currently available for patients with R/R MCL. These results can provide useful insights to clinicians and reimbursement decision-makers on the comparative effectiveness of ibrutinib enabling optimal treatment decisions to be made for patients with R/R MCL in clinical practice.
Figure 1: Predicted PFS (A) and OS (B) for R/R patients with MCL from the Lyon-Sud RW cohort if treated with ibrutinib versus physician's choice regimen
Sarkozy: Celgene: Honoraria; Roche/Genetech: Consultancy, Honoraria, Research Funding. Bachy: Amgen: Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding; Sandoz: Consultancy, Honoraria; Mundipharma: Research Funding. Karlin: Janssen: Honoraria, Other: Travel expenses. Ghesquières: Celgene and Mundipharma: Consultancy, Honoraria; Roche: Research Funding. Besson: Janssen Pharmaceutica NV: Other: employee of Janssen Pharmaceutica NV. Hermans: Janssen Pharmaceutica NV: Other: employee of QuintilesIMS, which is the vendor paid by Janssen Pharmaceutica NV to perform the extracting/cleaning/harmonization of the data. Healy: Janssen Pharmaceutica NV: Other: employee of Janssen Pharmaceutica NV. Garside: Janssen Pharmaceutica NV: Other: employee of Janssen Pharmaceutica NV. Diels: Janssen Pharmaceutica NV: Other: employee and equity owner of Janssen Pharmaceutica NV. MacDougall: Janssen Pharmaceutica NV: Other: employee of QuintilesIMS, which is the vendor paid by Janssen Pharmaceutica NV to perform the extracting/cleaning/harmonization of the data. Pick-Lauer: Janssen Pharmaceutica NV: Other: employee of Janssen Pharmaceutica NV. Salles: Mundipharma: Honoraria; Gilead: Honoraria, Research Funding; Janssen, Celgene, Novartis, and Amgen: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal